Healthcare Alerts | Infectious Diseases | MPox
DRC launches Mpox vaccination campaign as WHO approves new diagnostic test to boost global response
Time to read: 02:27 minutes
Time to listen: 05:09 minutes
Type of article: News
MedED Catalogue Reference: MNMP048
Category: News
Category Cross-reference: Infectious Diseases, Global Health
Keywords: WHO, Mpox, vaccinations, DRC
Top
6 October 2024 13:30
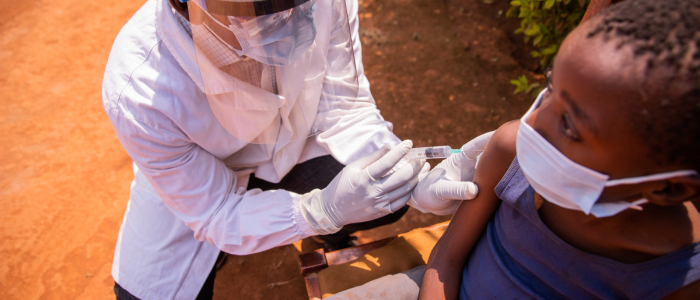
The Democratic Republic of the Congo (DRC) has initiated a crucial mpox vaccination campaign aimed at curbing the ongoing outbreak of the viral disease.
The vaccination rollout, which began in the eastern North Kivu province, is part of broader efforts to contain the spread of mpox and protect frontline health workers and other at-risk groups. With over 30,000 suspected and confirmed cases reported since the start of 2024, the DRC accounts for 90% of mpox cases across 15 African countries, making this intervention essential.
Health authorities have received 265,000 doses of the MVA-BN vaccine, donated by partners including the European Commission’s Health Emergency Preparedness and Response Authority, Gavi, the Vaccine Alliance, and the U.S. Government. The campaign prioritizes healthcare workers, contacts of confirmed cases, and other vulnerable groups and will expand to 11 high-incidence health zones across six provinces.
“This marks an important step in limiting the spread of the virus and ensuring the safety of communities,” said Dr. Matshidiso Moeti, WHO Regional Director for Africa, emphasizing the importance of vaccines in outbreak control.
In parallel, the World Health Organization (WHO) has made a major breakthrough in expanding mpox diagnostic capabilities.On October 3, WHO listed the first mpox in vitro diagnostic (IVD) test under its Emergency Use Listing (EUL) procedure.
The Alinity m MPXV assay, developed by Abbott Molecular Inc., is a real-time PCR test designed to detect mpox virus DNA from skin lesion swabs, offering a critical tool in rapid diagnosis.
With limited testing capacity, particularly in African nations, this new diagnostic approval is expected to enhance early detection and containment efforts significantly. Only 37% of suspected mpox cases in the DRC have been tested this year, underscoring the urgent need for expanded diagnostic access.
“This first diagnostic test represents a significant milestone in expanding testing availability in affected countries,” said Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products. By enabling quicker diagnosis, the Alinity m MPXV assay will help health workers efficiently confirm mpox cases, improving outbreak control.
The new test joins WHO's broader efforts to address the global mpox outbreak. WHO has called on manufacturers to submit additional diagnostic tests for emergency use listing, aiming to expand the range of testing options available to countries struggling to contain the virus.
In the DRC, WHO’s support has gone beyond vaccination and diagnostics. More than 300 WHO experts originally engaged in polio eradication efforts have been integrated into the mpox response, assisting with active surveillance, case investigations, and risk communication. These public health measures, combined with vaccines and diagnostics, form a comprehensive approach to curbing the spread of the virus.
As the DRC continues its vaccination efforts and benefits from improved testing, WHO and its partners are working closely with national authorities to ensure that resources are effectively deployed, with a focus on community engagement and combating vaccine misinformation.
These advances—both in vaccination and diagnostics—are critical steps in the global response to mpox, especially in Africa where cases continue to rise. With WHO’s prequalification of the MVA-BN vaccine and the approval of the first emergency-use diagnostic test, there is hope that access to life-saving tools will improve, helping to contain the outbreak and protect vulnerable populations.
5 October 2024 | WHO | The Democratic Republic of the Congo kicks off mpox vaccination
3 October 2024 | WHO| WHO approves first mpox diagnostic test for emergency use, boosting global access
Access more articles related to Mpox
23 September 2024 India reports first case of mpox infection from clade 1b variant
16 September 2024 | Deliveries, approvals, and collaboration - Momentum builds in Africa’s Mpox fight
13 September 2024 | The total number of Mpox cases reaches 25 as DoH confirms new case in Western Cape
Access all Mpox articles and resources
Back to top
Disclaimer
This article is compiled from various resources researched and compiled by the contributor. It is in no way presented as an original work. Every effort has been made to correctly attribute quotes and content. Where possible all information has been independently verified. The Medical Education Network bears no responsibility for any inaccuracies which may occur from the use of third-party sources. If you have any queries regarding this article contact us
Fact-checking Policy
The Medical Education Network makes every effort to review and fact-check the articles used as source material in our summaries and original material. We have strict guidelines in relation to the publications we use as our source data, favouring peer-reviewed research wherever possible. Every effort is made to ensure that the information contained here is an accurate reflection of the original material. Should you find inaccuracies, out of date content or have any additional issues with our articles, please make use of the contact us form to notify us.


