Clinical Summary | Infectious Diseases, Vaccines & Antimicrobials
Antivirals for treatment of severe influenza: a systematic review and network meta-analysis of randomised controlled trials
Time to read: 10: 49
Originally Published: 23 August 2024
Source: The Lancet
Type of article: Clinical Research Summary
MedED Catalogue Reference: MICS005
Category: Infectious Diseases, Vaccines & Antimicrobials
Cross-reference: Critical Care
Keywords: influenza, antivirals
Originally Published: The Lancet 23 August 2024. This is a summary of the clinical study, reproduced under Creative Commons Attribution (CC BY 4.0) and in no way represents the original research. Links to all original material can be found at the end of this summary.
Key Take Aways
1. Mortality rates in patients hospitalised patients with severe influenza can be as high as 10-15%
2. Antiviral treatments like oseltamivir and peramivir may reduce the duration of hospitalisation compared to standard care or placebo, but the evidence supporting this benefit is of low certainty.
3. The overall impact of antiviral treatments on mortality and other critical patient outcomes remains uncertain due to limited data from randomised controlled trials.
Top
Overview | Objectives | Study Design | Findings | Discussion| Limitations | Conclusion | Original Research | Funding | References
Overview
Influenza typically presents with mild to moderate upper respiratory symptoms that resolve within a week. However, certain populations, including young children under five, older adults aged 65 and above, pregnant women, and individuals with chronic medical conditions, are at an increased risk of developing severe illness from the infection.
In patients hospitalised with seasonal influenza, complications such as severe pneumonia, respiratory failure, multi-organ failure, and secondary bacterial infections can lead to significant mortality, with case-fatality rates for adults ranging from 4% to 8%. Among immunocompromised individuals, the mortality rate can be as high as 10% to 15%.
Therefore, identifying effective therapies for severe influenza is essential for global public health.
Antivirals, particularly neuraminidase inhibitors, are recommended for patients with severe influenza. Systematic reviews and meta-analyses suggest that these treatments, when administered early, may reduce mortality and shorten hospital stays. However, most studies have primarily focused on the relative effects of neuraminidase inhibitors, neglecting to assess the impact of antivirals on zoonotic influenza or to evaluate the certainty of the evidence.
Back to top
Study Purpose
In this study, researchers of this study, Gao & Guyatt et al. aimed to determine the optimal antiviral drug for the treatment of severe influenza and to support the updated WHO influenza guidelines (September 13, 2024).
Back to top
Study Design & Selection Criteria
The search included randomised controlled trials published up to September 20, 2023.
Trial inclusion criteria
Trials that enrolled hospitalised patients with suspected or confirmed influenza and compared direct-acting influenza antivirals against placebo, standard care, or other antivirals were included.
The following databases were included in the systematic search:
Embase, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature, Global Health, Epistemonikos, and ClinicalTrials.gov.
Data Collection:
The certainty of evidence was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
Back to top
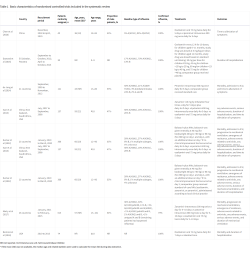
Eight trials with 1424 participants (mean age 36–60 years for trials that reported mean or median age; 43–78% male patients) were included in this systematic review, of which six were included in the network meta-analysis.1-8
The interventions in the trials included oseltamivir, peramivir, zanamivir, rimantadine, zanamivir combined with rimantadine, and baloxavir combined with neuraminidase inhibitors.
Three trials directly compared oseltamivir and peramivir to standard care or placebo.2,3,8
The remaining five trials compared different antivirals:
-
One trial compared oseltamivir with zanamivir 7
-
One compared zanamivir and rimantadine with rimantadine alone 4
- One trial compared baloxavir plus neuraminidase inhibitors with neuraminidase inhibitors alone
Table 1 below details the basic characteristics of the randomised controlled trials included in this systematic review
Click to enlarge the image
Back to top
Findings
A network meta-analysis encompassing four trials evaluated the impact of oseltamivir, peramivir, and zanamivir on mortality among 813 patients with severe seasonal influenza.
The findings revealed a range of risk differences, indicating that these antiviral agents could potentially decrease mortality by up to 18 fewer deaths per 1,000 patients for seasonal influenza and as much as 232 fewer deaths per 1,000 for zoonotic influenza. However, some comparisons indicated a slight increase in risk, although the certainty of this evidence remains very low. 4,5,6,7,8
In a separate analysis focused on ICU admissions, two trials involving 235 patients with severe seasonal influenza compared the effects of oseltamivir and peramivir.3,5
- The risk differences for ICU admission varied, showing 29 fewer to 43 more admissions per 1,000 patients, again with very low certainty in the evidence.
Three trials, which included a total of 226 patients suffering from severe seasonal influenza, reviewed the hospital duration difference between patients treated with either oseltamivir or peramivir. Results indicated that oseltamivir reduced hospitalisation by an average of 1.63 days compared to standard care or placebo, while peramivir led to a reduction of 1.73 days, though again, the evidence supporting these findings is classified as low certainty.2,5,8
Two trials involving a total of 752 patients assessed adverse events associated with oseltamivir, peramivir, and zanamivir in severe influenza cases.
- The analysis showed no significant differences in the occurrence of either adverse or serious adverse events among the three antivirals. 5,7
One study specifically examined the outcomes related to oseltamivir or zanamivir treatment, focusing on progression to mechanical ventilation, duration of mechanical ventilation, emergence of resistance, and associated adverse events.
- Compared to zanamivir, oseltamivir presented relative risks ranging from 1.20 to 2.89 for these outcomes, with 95% confidence intervals overlapping the null effect. The average duration of mechanical ventilation was 0.89 days longer with oseltamivir, reinforcing the very low certainty of this evidence.7
Researchers compared the combination of baloxavir and neuraminidase inhibitors to neuraminidase inhibitors alone.
- This study found minimal differences in hospitalisation duration (with a mean difference of 0.31 days shorter) and a slight reduction in the emergence of antiviral resistance (25 fewer cases per 1,000 patients), both supported by low certainty evidence. Very low certainty evidence also surrounded the effects of combination treatment on ICU admissions, mechanical ventilation, mortality, or adverse events when compared to neuraminidase inhibitors alone.6
Finally, a single trial explored the effects of combining zanamivir with rimantadine against rimantadine alone. However, the evidence regarding this combination's impact on hospitalisation duration, mortality, and adverse events was categorised as very low certainty. 4
Discussion
Antivirals play a crucial role in managing severe influenza, with previous studies suggesting that early treatment with neuraminidase inhibitors may lower mortality and shorten hospital stays.
However, these analyses primarily focused on neuraminidase inhibitors, did not address severe zoonotic influenza, and lacked an evaluation of evidence certainty.
To date, no network meta-analysis has comprehensively assessed all antiviral treatments for severe influenza, leaving the optimal antiviral drug uncertain.
This study presents low-certainty evidence indicating that oseltamivir and peramivir may reduce the duration of hospitalisation for patients with severe seasonal influenza compared to placebo or standard care.
Despite this, there remains significant uncertainty about the impact of oseltamivir, peramivir, and zanamivir on mortality rates in both seasonal and zoonotic influenza cases.
Importantly, no significant differences were found in adverse or serious adverse events among the three antivirals, although this conclusion is based on very low certainty evidence. Additionally, the effects of other antivirals like baloxavir on mortality and other critical outcomes in severe influenza patients remain unclear.
The implications of this evidence highlight that while oseltamivir and peramivir may shorten hospitalisation duration, the overall uncertainty regarding antiviral effectiveness persists. These findings support the continued use of these antivirals but underscore the need for further clinical trials to clarify their benefits, safety, and potential impacts on antiviral resistance in patients with severe influenza.
Back to top
Limitations
The researchers indicate that the study had a number of limitations including, including that only eight eligible trials were identified, with six included in the network meta-analyses, leading to uncertainty about the effects of antivirals on severe influenza outcomes.
Sparse data prevented subgroup analyses based on secondary bacterial infections or influenza type, and there was a lack of information on individuals over 60 and children. Additionally, most trials focused on seasonal influenza, raising concerns about bias from inadequate allocation concealment and blinding. Consequently, the certainty of evidence for all comparisons was assessed as low or very low.
Future trials may enhance evidence quality, prompting periodic updates to this systematic review.
Back to top
Conclusion
These findings support the continued use of these antivirals but underscore the need for further clinical trials to clarify their benefits, safety, and potential impacts on antiviral resistance in patients with severe influenza.
The study is registered with PROSPERO under the identifier CRD42023456650.
Access the original research
Gao, Y., Guyatt, G., Uyeki, T. M., Liu, M., Chen, Y., Zhao, Y., Shen, Y., Xu, J., Zheng, Q., Li, Z., Zhao, W., Luo, S., Chen, X., Tian, J., & Hao, Q. (2024). Antivirals for treatment of severe influenza: a systematic review and network meta-analysis of randomised controlled trials. Lancet (London, England), 404(10454), 753–763. https://doi.org/10.1016/S0140-6736(24)01307-2
Reproduced under Creative Commons CC-BY License
Conflict of Interest, Funding and Support
Role of the Funder/Sponsor
The study's funder had no role in the design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest Disclosures
The researchers declared that there were no conflicts of interests
Funding/Support
The World Health Organisation.
This study was reproduced under Creative Commons CC-BY licence.
References
1. Chen, H. D., Wang, X., Yu, S. L., Ding, Y. H., Wang, M. L., & Wang, J. N. (2020). Clinical Effectiveness of Intravenous Peramivir Compared With Oseltamivir in Patients With Severe Influenza A With Primary Viral Pneumonia: A Randomized Controlled Study. Open forum infectious diseases, 8(1), ofaa562. https://doi.org/10.1093/ofid/ofaa562
2. Dawood, F. S., Jara, J., Gonzalez, R., Castillo, J. M., De León, T., Estripeaut, D., Luciani, K., Sujey Brizuela, Y., Barahona, A., Cazares, R. A., Lawson, A. M., Rodriguez, M., de Viana, D., Franco, D., Castillo, M., Fry, A. M., Gubareva, L., Tamura, D., Hughes, M., Gargiullo, P., … Widdowson, M. A. (2016). A randomized, double-blind, placebo-controlled trial evaluating the safety of early oseltamivir treatment among children 0-9 years of age hospitalized with influenza in El Salvador and Panama. Antiviral research, 133, 85–94. https://doi.org/10.1016/j.antiviral.2016.07.007
3. de Jong, M. D., Ison, M. G., Monto, A. S., Metev, H., Clark, C., O'Neil, B., Elder, J., McCullough, A., Collis, P., & Sheridan, W. P. (2014). Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 59(12), e172–e185. https://doi.org/10.1093/cid/ciu632
4. Ison, M. G., Gnann, J. W., Jr, Nagy-Agren, S., Treannor, J., Paya, C., Steigbigel, R., Elliott, M., Weiss, H. L., Hayden, F. G., & NIAID Collaborative Antiviral Study Group (2003). Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antiviral therapy, 8(3), 183–190.
5. Ison, M. G., Hui, D. S., Clezy, K., O'Neil, B. J., Flynt, A., Collis, P. J., Simon, T. J., & Alexander, W. J. (2013). A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antiviral therapy, 18(5), 651–661. https://doi.org/10.3851/IMP2442
6. Kumar, D., Ison, M. G., Mira, J. P., Welte, T., Hwan Ha, J., Hui, D. S., Zhong, N., Saito, T., Katugampola, L., Collinson, N., Williams, S., Wildum, S., Ackrill, A., Clinch, B., & Lee, N. (2022). Combining baloxavir marboxil with standard-of-care neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. The Lancet. Infectious diseases, 22(5), 718–730. https://doi.org/10.1016/S1473-3099(21)00469-2
8. Ramirez, J., Peyrani, P., Wiemken, T., Chaves, S. S., & Fry, A. M. (2018). A Randomized Study Evaluating the Effectiveness of Oseltamivir Initiated at the Time of Hospital Admission in Adults Hospitalized With Influenza-Associated Lower Respiratory Tract Infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 67(5), 736–742. https://doi.org/10.1093/cid/ciy163
Back to top
Disclaimer
This article is in no way presented as an original work. Every effort has been made to attribute quotes and content correctly. Where possible, all information has been independently verified. The Medical Education Network bears no responsibility for any inaccuracies which may occur from the use of third-party sources. If you have any queries regarding this article contact us
Fact-checking Policy
The Medical Education Network makes every effort to review and fact-check the articles used as source material in our summaries and original material. We have strict guidelines in relation to the publications we use as our source data, favouring peer-reviewed research wherever possible. Every effort is made to ensure that the information contained here accurately reflects the original material. Should you find inaccuracies or out-of-date content or have any additional issues with our articles, please make use of the Contact Us form to notify us.