Clinical Summaries | Oncology, Radiotherapy, Women's Health
Oncology & Radiotherapy: Adjuvant Chemoradiation vs Radiotherapy in Stage I–IIA Cervical Cancer Post-Hysterectomy | Results from the NRG Oncology/GOG-263 (KGOG 1008) Trial
4 February 2026
The NRG Oncology/GOG-263 (KGOG 1008) trial found that
Quick Facts
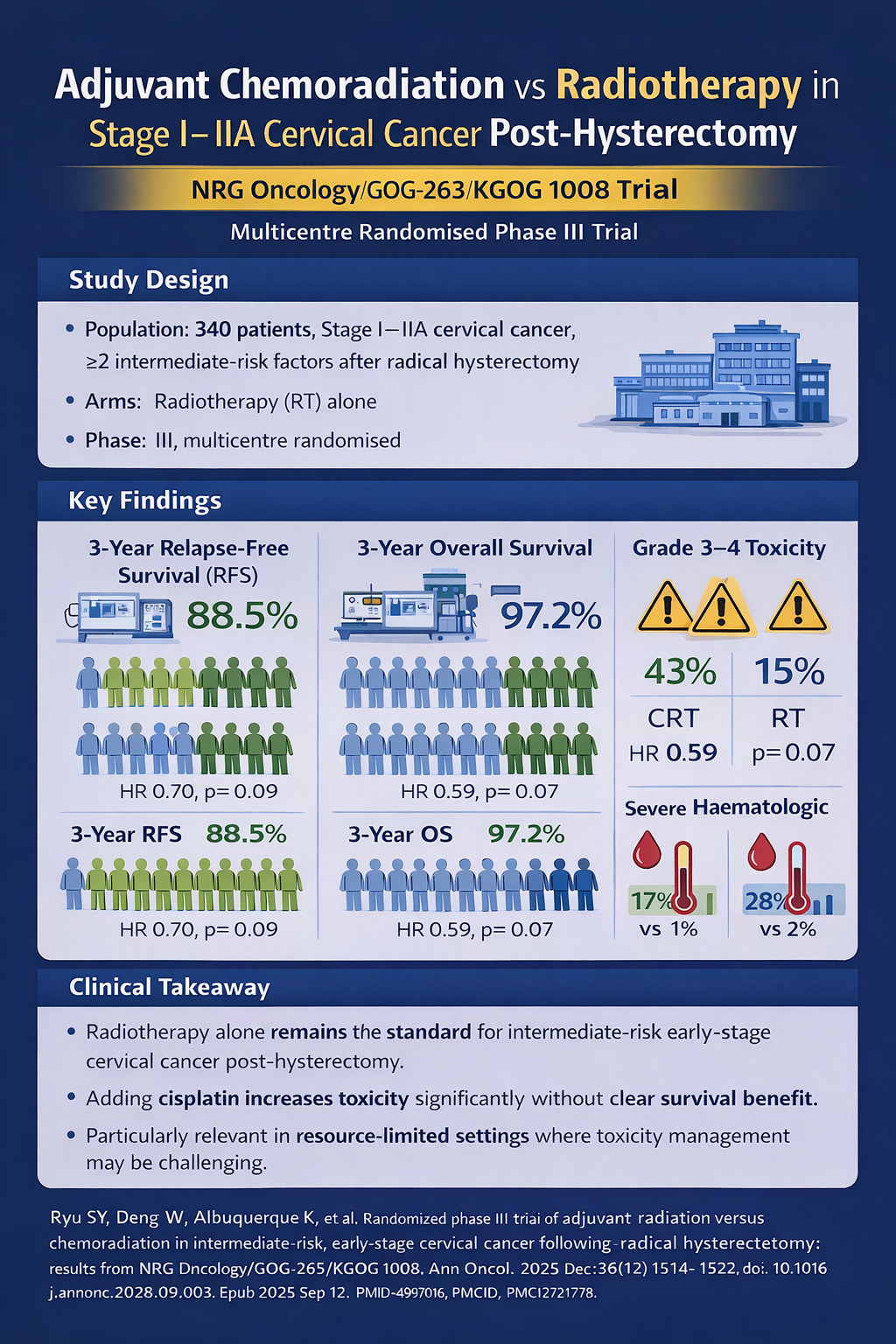
- Design: Multicentre randomised phase III trial
- Population: 340 patients with stage I–IIA cervical cancer and ≥2 intermediate-risk factors post-radical hysterectomy
- Arms: Adjuvant RT alone vs concurrent chemoradiation (weekly cisplatin); IMRT allowed
- Key finding: Chemoradiation showed non-significant trends towards improved RFS and OS but caused markedly higher toxicity
- Outcomes:
- 3-year RFS: 88.5% (CRT) vs 85.4% (RT); HR 0.70, p=0.09
- 3-year OS: 97.2% (CRT) vs 90.3% (RT); HR 0.59, p=0.07
- Grade 3–4 toxicity: 43% (CRT) vs 15% (RT)
- Severe haematologic toxicity: neutropenia 17% vs 1%; leucopenia 28% vs 2%
- Clinical takeaway:
Radiotherapy alone remains the standard for intermediate-risk early-stage cervical cancer post-hysterectomy. Adding cisplatin increases toxicity without a clear survival benefit — a key consideration in resource-limited settings.
Top
Study Context
The management of early-stage cervical cancer with intermediate-risk factors following radical hysterectomy has historically relied on adjuvant radiotherapy (RT) alone as standard treatment. Intermediate-risk criteria (Sedlis criteria) include tumour size, depth of stromal invasion, and lymphovascular space involvement.
Whilst chemoradiation has become established as superior therapy for locally advanced cervical cancer and high-risk early-stage disease, evidence supporting the addition of chemotherapy to adjuvant radiation in intermediate-risk patients remained limited.
The landmark GOG-92 trial, conducted 25 years prior, demonstrated benefit from RT alone but did not include chemotherapy. Given advances in radiation techniques, imaging modalities, and supportive care, along with clinical equipoise regarding whether adding cisplatin chemotherapy could improve outcomes, this trial was designed to definitively answer whether chemoradiation provides superior efficacy to justify potential increased toxicity.
Study Objective
The NRG Oncology/GOG-263 (KGOG 1008)
Study Methods
The trial enrolled 340 women with stage I-IIA cervical cancer who underwent radical hysterectomy with bilateral pelvic lymph node dissection and had at least two intermediate-risk pathological factors.
Patients were randomised to receive either RT alone (172 patients) or concurrent chemoradiation (168 patients) with weekly cisplatin at 40 mg/m² for up to six cycles. Intensity-modulated radiation therapy was permitted in both arms.
The efficacy analysis included 158 patients per arm with balanced baseline characteristics including performance status, histology, tumour grade, age (median 45-46.5 years), and ethnicity (50-55% Asian).
Study Findings
The trial demonstrated trends towards improved outcomes with chemoradiation that did not reach statistical significance.
Three-year recurrence-free survival was 88.5% with chemoradiation versus 85.4% with RT alone (HR 0.70, p=0.09). Three-year overall survival favoured chemoradiation at 97.2% versus 90.3% (HR 0.59, p=0.07). However, chemoradiation significantly increased grade 3-4 toxicities (43% vs 15%), particularly neutropenia (17% vs 1%) and leucopenia (28% vs 2%).
Approximately 91% of patients in the chemoradiation arm completed at least four cycles of weekly cisplatin.
Patient-reported outcomes indicated transient quality-of-life decrements with chemoradiation. Subgroup analyses suggested potential benefits with modern radiation techniques like IMRT.
Clinical Interpretation
This superiority trial failed to demonstrate statistically significant improvements in recurrence-free survival or overall survival with the addition of weekly cisplatin to adjuvant radiation therapy in intermediate-risk cervical cancer patients. The increased toxicity burden, including significantly higher rates of severe haematologic adverse events and temporary quality-of-life impairment, was not offset by definitive survival benefits.
These findings support continuing radiotherapy alone as the standard adjuvant treatment for patients with intermediate-risk early-stage cervical cancer following radical hysterectomy. Future research should investigate whether technological advances in radiation delivery might improve outcomes in this population. Patient selection criteria may also influence treatment benefit, suggesting higher-risk subgroups might derive greater advantage from combined modality therapy.
Why This is Relevant for South African Practitioners
Cervical cancer is the fourth most common cancer amongst women worldwide, with the highest incidence in low-income countries, particularly in sub-Saharan Africa, where almost 90% of cervical deaths occur in resource-constrained settings. Early cervical cancer screening can detect cervical intraepithelial neoplasia grades 2 or 3—precancerous lesions that, if detected, treated, and managed early, can prevent the development of invasive cervical cancer.
The findings from the NRG Oncology/GOG-263/KGOG 1008 trial hold particular significance for South Africa, where cervical cancer remains a critical public health challenge despite being largely preventable.
On February 2, 2026, the Department of Health launched the 2026 Human Papillomavirus (HPV) Vaccination drive targeting girls aged nine years and older, aiming to protect future generations from developing cervical cancer. This primary prevention initiative comes against the backdrop of alarming statistics: South Africa records over 5,700 new cervical cancer cases annually, with more than 3,000 women dying from cervical cancer-related complications each year.1
South Africa's cervical cancer burden far exceeds global averages. The age-standardised incidence rate stands at 31.7 per 100,000 persons compared to 14.0 per 100,000 globally, whilst the age-standardised mortality rate reaches 18 per 100,000 women versus 6.8 per 100,000 internationally. Cervical cancer ranks as the second most common cancer amongst South African women and the leading cause of cancer death across all age groups. The disease disproportionately affects black women (82.7% of cases) and represents the most common malignancy in women aged 15-44 years. 2,3
The HIV epidemic significantly compounds this burden, as cervical cancer was classified as an AIDS-defining illness in 1993. Women living with HIV face substantially higher risks of HPV-associated disease and more aggressive cervical cancer progression, making unvaccinated HIV-positive girls and women particularly vulnerable to serious health complications. The epidemic has also diverted limited healthcare resources away from preventive activities like cancer screening, exacerbating detection delays.2,3
For the subset of South African women diagnosed with early-stage cervical cancer who undergo radical hysterectomy and are found to have intermediate-risk pathological features, the GOG-263 trial results provide crucial treatment guidance—but equally important is the question of whether adding chemotherapy to radiation is even practical in resource-constrained settings.
The finding that radiotherapy alone achieves outcomes comparable to more toxic chemoradiation has important resource implications for South Africa's healthcare system. In settings where managing chemotherapy-related complications (particularly severe neutropenia and leucopenia requiring supportive care, blood products, and antimicrobial therapy) poses significant logistical and financial challenges, evidence supporting RT alone as standard treatment offers a more feasible approach without compromising patient outcomes.
This is particularly relevant given that 43% of patients receiving chemoradiation experienced grade 3-4 toxicities compared to only 15% with RT alone.
However, the broader message remains clear: primary prevention through HPV vaccination and early detection through screening programmes represent the most effective strategies to reduce South Africa's disproportionate cervical cancer burden. The current vaccination campaign, combined with evidence-based treatment protocols for those who do develop disease, offers a comprehensive approach to addressing this preventable cause of premature death amongst South African women.
Original Study
References
1. South African Department of Health. (2026, February 2). Health on Human Papilloma Virus vaccination campaign to protect girls from cervical cancer [Press release].
2. Dennyi, L. (2017). Cervical cancer prevention and early detection from a South African perspective. In South African Health Review: 20 Year Anniversary Edition (pp. 189-196). Health Systems Trust
3. Ngambi, D., & Ramathuba, D. U. (2024). Challenges regarding the implementation of cervical cancer screening guidelines in Limpopo province, South Africa. African journal of primary health care & family medicine, 16(1), e1–e7. https://doi.org/10.4102/phcfm.v16i1.4487
Disclaimer:
The content in this summary is intended as an overview only and does not replace the original research. Members should review the original study before forming clinical opinions. The Medical Education Network cannot be held liable for inaccuracies or omissions.
Fact-checking Policy:
The Medical Education Network makes every effort to review and fact-check source material. Please use the contact us form to report issues


