Escalating rifampin to 35 mg/kg daily provided no survival advantage in adult TBM patients. Mortality at 6 months remained comparable across both treatment groups (approximately 45% vs 41% in the high-dose and standard-dose arms, respectively), while hepatotoxicity nearly doubled with intensified therapy. In a cohort where 61% were HIV-positive, these findings underscore that rifampin monointensification is inadequate.
In Brief | Infectious Diseases, Neurology
Infectious Diseases: Escalating Rifampin Dose Fails to Reduce Mortality in Tuberculous Meningitis
26 January 2026
In adults with tuberculous meningitis, high-dose oral rifampin failed to reduce mortality and may increase harm, despite a strong pharmacological rationale.
Quick Facts
- Condition: Adult tuberculous meningitis
- Study type: Double-blind, randomised, placebo-controlled trial
- Participants: 499 adults; 61% living with HIV
- Intervention: High-dose oral rifampin (35 mg/kg/day) for 8 weeks
- Comparator: Standard-dose rifampin (10 mg/kg/day)
- Primary outcome: 6-month all-cause mortality
- Result: No mortality benefit with high-dose rifampin
- Safety signal: Higher rates of drug-induced liver injury with high-dose therapy
Top
Study Objective
To determine whether high-dose rifampin improves survival in adults with tuberculous meningitis, including both HIV-positive and HIV-negative patients, by increasing cerebrospinal fluid drug concentrations.
Study Summary
Tuberculous meningitis ranks among tuberculosis's most lethal manifestations, with early mortality rates in African settings reaching 25-70%. In South Africa, HIV co-infection compounds this burden—even survivors frequently face severe neurological disability despite optimal antimicrobial therapy.
The challenge lies partly in pharmacokinetics: rifampin achieves suboptimal cerebrospinal fluid concentrations at standard dosing, raising the question of whether dose escalation could enhance CNS penetration and improve outcomes.
This multicentre, double-blind, randomised, placebo-controlled trial addressed that question directly, enrolling 499 adults with definite or probable TBM across Indonesia, South Africa, and Uganda. The cohort reflected the HIV-TB syndemic reality, with 61% of participants living with HIV.
The findings appeared in the New England Journal of Medicine in December 2025.
Study Design
All participants received standard four-drug therapy (isoniazid, rifampin 10 mg/kg/day, ethambutol, pyrazinamide).
The intervention arm received supplemental rifampin to achieve 35 mg/kg/day for 8 weeks; controls received a matched placebo. Both groups continued standard therapy through completion of the 9-12 month treatment course. The cohort included 304 (61%) persons living with HIV.
The primary outcome was all-cause mortality at 6 months, chosen to capture deaths occurring during the early, high-risk phase of TBM.
Secondary outcomes included adverse events, particularly drug-induced liver injury.
Study Findings
The trial revealed no survival benefit from dose intensification.
Six-month mortality was statistically equivalent between arms: 44.6% (n=109) with high-dose rifampin versus 40.7% (n=100) on standard therapy (HR 1.17, 95% CI 0.89-1.54, P=0.25).
More concerning, deaths occurred earlier with intensified treatment—median 13 days (IQR 4-39) compared to 24 days (IQR 6-56) in controls. Hepatotoxicity rates nearly doubled (8.0% vs 4.4%), although no deaths resulted from liver injury.
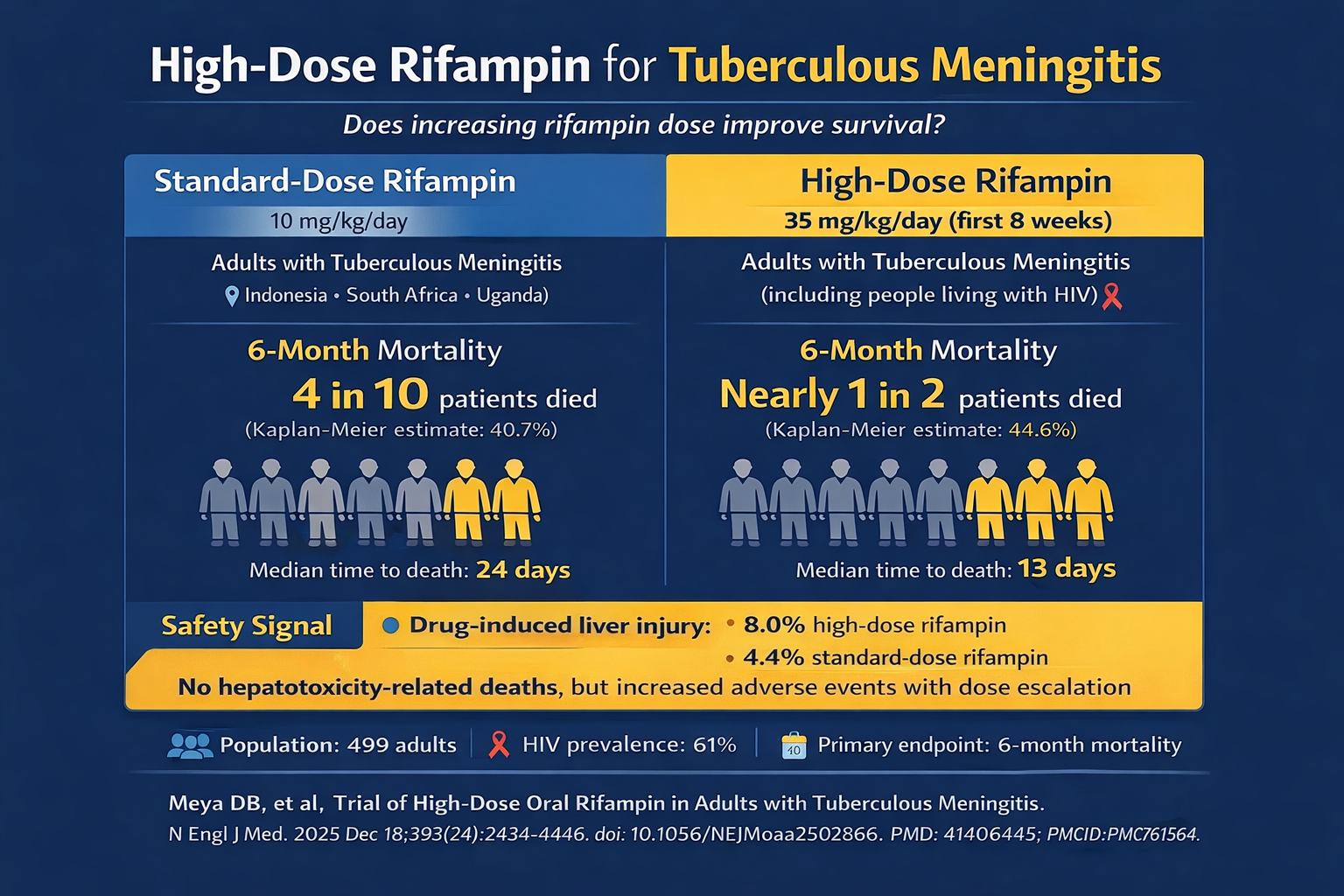
Escalating rifampin to 35 mg/kg daily provided no survival advantage in adult TBM patients. Mortality at 6 months remained comparable across both treatment groups (approximately 45% vs 41% in the high-dose and standard-dose arms, respectively), while hepatotoxicity nearly doubled with intensified therapy. In a cohort where 61% were HIV-positive, these findings underscore that rifampin monointensification is inadequate.
Why is this Relevant for South African Practitioners
Original Study
References
The content in this summary is intended as an overview only and does not replace the original research. Members should review the original study before forming clinical opinions. The Medical Education Network cannot be held liable for inaccuracies or omissions.
Fact-checking Policy:
The Medical Education Network makes every effort to review and fact-check source material. Please use the contact us form to report issues.


